 This is a second article in the series on the principles of formulating water-compatible cannabis extracts and isolates, also known as water-soluble CBD and THC. The first article showed multiple advantages of nanoemulsions over the other two water-compatible formulation classes: microemulsions and liposomes. Here I will demonstrate the importance of using a carrier oil in your cannabis extract or isolate nanoemulsion. I will also explain how to select the proper carrier oil among the available choices.
This is a second article in the series on the principles of formulating water-compatible cannabis extracts and isolates, also known as water-soluble CBD and THC. The first article showed multiple advantages of nanoemulsions over the other two water-compatible formulation classes: microemulsions and liposomes. Here I will demonstrate the importance of using a carrier oil in your cannabis extract or isolate nanoemulsion. I will also explain how to select the proper carrier oil among the available choices.
Why do I need a carrier oil in my water-soluble CBD or THC nanoemulsion?
As explained in my earlier article, there are three main reasons to formulate cannabis extracts and isolates as nanoemulsions:
- Compatibility with water: readily mixable with any beverage.
- High bioavailability:
factor of 9 enhancement was reported for similar compounds [1]. - Long-term stability (shelf life): stable for up to several years [2].
While a cannabis extract or isolate emulsion is possible to produce without the use of any carrier oil, it will always be unstable and poorly bioavailable. Such emulsions suffer from:
- Relatively large droplet sizes: reduced stability and bioavailability.
- Ostwald ripening: reduced stability.
- Lack of long-chain free fatty acid source: reduced bioavailability.
A correctly chosen carrier oil is essential for improving all of the above. Let’s go through these improvements step-by-step.
Droplet size reduction: improves stability and bioavailability
As explained earlier, nanoemulsions must have small droplet sizes (< 300 nm) in order to be kinetically stable [3] and provide high bioavailability [1]. It is well-known in the pharmaceutical and food industries that the smallest nanoemulsion droplet size is achieved when the dispersed phase (oil) and
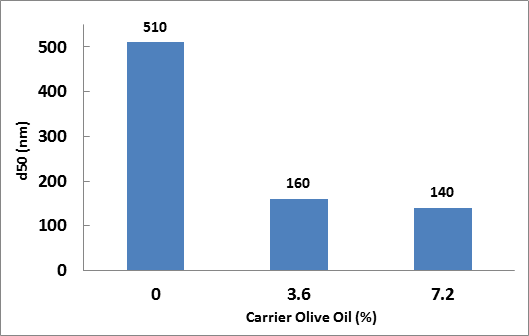 |
| Figure 1. The effect of olive carrier oil on the median droplet size (d50) of a CBD extract nanoemulsion produced with the BSP-1200 ultrasonic processor. The following concentrations were constant: Tween 80 = 3.85 %, Span 80 = 0.65 % (total surfactant = 4.5 %, HLB = 13.5), CBD extract = 5.4% (54 mg/ml). |
Ostwald ripening prevention: improves stability
Ostwald ripening is a destabilization process, through which larger oil droplets in a nanoemulsion grow at the expense of smaller ones. This occurs because the difference in Laplace pressure causes smaller oil droplets to have higher local solubilities than the larger ones [6]. The rate of this process is mainly defined by the aqueous solubility of the oil [7]: the quicker the oil is able to diffuse between the droplets through the water phase, the faster will be the Ostwald ripening process. The solubility of cannabinoids in water averages to approximately 0.0015 mg/ml [8], which is too low for efficient delivery into the body, but high enough to cause rapid nanoemulsion destabilization through Ostwald ripening.
For a nanoemulsion of C8 MCT oil (tricaprylin) with an aqueous solubility of 0.0004 mg/ml [9], the average droplet diameter has been shown to double every 18.3 hours [7]. Since their solubilities are even higher, this rate will be greater for CBD or THC emulsified without a correctly selected carrier oil. The issue, however, can be resolved by the introduction of 50 % or more of a water-insoluble LCT carrier oil (e.g., olive oil) into the dispersed (oil) phase, which brings the rate of Ostwald ripening down to a practically undetectable level [7].
Long-chain free fatty acid source: improves bioavailability
 The function of carrier oils is not limited to helping produce stable nanoemulsions with small droplet sizes - they are also essential for maximizing the intestinal uptake. A number of studies have demonstrated that carrier oil type has a major influence on the bioavailability of poorly water-soluble drugs incorporated in nanoemulsion-based delivery systems. The highest bioavailability after oral administration is obtained with LCT (e.g., olive, corn or fish oil), drastically diminishing with MCT (e.g., fractionated coconut oil) and non-triglyceride oils (e.g., orange or mineral oil) [10, 11]. The underlying mechanism has to do with the way poorly water-soluble compounds are transported across the epithelial tissue in the small intestine.
The function of carrier oils is not limited to helping produce stable nanoemulsions with small droplet sizes - they are also essential for maximizing the intestinal uptake. A number of studies have demonstrated that carrier oil type has a major influence on the bioavailability of poorly water-soluble drugs incorporated in nanoemulsion-based delivery systems. The highest bioavailability after oral administration is obtained with LCT (e.g., olive, corn or fish oil), drastically diminishing with MCT (e.g., fractionated coconut oil) and non-triglyceride oils (e.g., orange or mineral oil) [10, 11]. The underlying mechanism has to do with the way poorly water-soluble compounds are transported across the epithelial tissue in the small intestine.
Correctly prepared nanoemulsions have been shown to remain stable in the presence of saliva and gastric fluids [11]. They are able to relatively quickly travel through the stomach into the small intestine, where they mix with lipases, phospholipids and bile salts released by the gallbladder. If the nanoemulsion incorporates a digestible carrier oil (MCT or LCT), the lipases convert it to free fatty acids. Together with the bile salts and phospholipids, the free fatty acids form mixed micelles, whose job it is to incorporate and actively transport hydrophobic nutrients (in this case, cannabinoids) through specific channels in the epithelial cells of the small intestine into the blood.
Indigestible carrier oils do not produce free fatty acids and, therefore, cannot properly contribute to the formation of mixed micelles. Among the digestible oils, LCTs outperform MCTs because of the difference in the lengths of the associated free fatty acids chains (C16 - C18 for LCTs versus C8 - C10 for MCTs). Long-chain free fatty acids form larger mixed micelles that can easier solubilize and incorporate the nutrients, resulting in greater ability to transport them into the blood and, therefore, helping them achieve much higher bioavailability [10].
How much carrier oil should I use?
As a general rule, the amount of carrier oil in your formulation should somewhat exceed that of the cannabis extract or isolate. 50 - 60% of LCT in the oil phase is generally sufficient to prevent Ostwald ripening [7] as well as to help achieve the smallest droplet size (Fig. 3) [5].
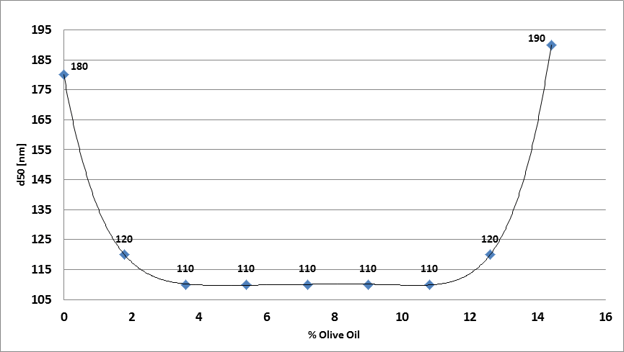 |
| Figure 3. The effect of olive oil concentration on the median particle size (d50) of a CBD extract nanoemulsion produced with the BSP-1200 ultrasonic processor. The following concentrations were constant: Quillaja Saponin from Q-Naturale = 4.5 % (based on 14 % Saponin content in Q-Naturale, HLB = 13.5), cannabis extract = 5.4% (54 mg/ml). |
What equipment can I use to produce CBD or THC nanoemulsions?
Laboratory, bench and industrial-scale ultrasonic liquid processors designed for the production of high-quality nanoemulsions are available from Industrial Sonomechanics. Our equipment makes it possible to:
- Turn cannabis concentrates and isolates into fully water-compatible nanoemulsions with enhanced bioavailability and rapid onset of action.
- Create all-natural or GRAS cannabinoid formulations that can be easily mixed into any beverage.
- Produce translucent CBD and THC nanoemulsions to infuse water with a strong dose of medicine while retaining optical clarity.
We also offer a new product called NanoStabilizer™, which is a proprietary blend of food-grade (GRAS) carrier oils, emulsifiers, and preservatives, and is used for the production of cannabis extract nanoemulsions (aka. water-soluble CBD or THC). You can find more information about this product and request a quotation here.
If you have any additional questions, please do not hesitate to contact us.
References:
[1] H. Yu, Q. Huang, Improving the oral bioavailability of curcumin using novel
[2] C. Solans, P. Izquierdo, J.
[3] T. Tadros, P. Izquierdo, J.
[4] D.J. McClements, Food Emulsions: Principles, Practices, and Techniques (2nd edition), CRC Press, Boca Raton, Florida, 2004.
[5] A. Peshkovsky, S. Leibtag, Data Obtained at Industrial Sonomechanics, LLC, (2016).
[6] L. Sagalowicz, M.E. Leser, Delivery systems for liquid food products, Curr. Opin. Colloid Interface Sci., 15 (2010) 61-72.
[7] T.J. Wooster, M. Golding, P. Sanguansri, Impact of oil type on nanoemulsion formation and Ostwald ripening stability, Langmuir, 24 (2008) 12758-12765.
[8] E.R. Garrett, C.A. Hunt, Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol, J. Pharm. Sci., 63 (1974) 1056-1064.
[9] N. Funasaki, H. Sakae, S. Keizo, The dissolution state of a triglyceride molecule in water and its orientation state at the air-water interface, Chem. Pharm. Bull., 24 (1976) 731-735.
[10] B. Ozturk, S.
[11] C. Qian, E.A. Decker, H. Xiao, D.J. McClements, Nanoemulsion delivery systems: Influence of carrier oil on b-carotene bioaccessibility, Food Chem., 135 (2012) 1440-1447.
.jpg?width=1994&height=332&name=Logo%20Sonomechanics%20White%20No%20Shadow%20R_Final%20(1).jpg)


