 Oil-in-water emulsions with nano-sized droplets (nanoemulsions) are widely used in the pharmaceutical industry, for example, as an intravenous source of fatty acids when oral nutrition is disadvantageous or as bioactive compound carriers (e.g. drugs, vaccines). Pharmaceutical nanoemulsions can be administered by almost all available routes including parenteral, ocular, nasal, oral, topical, and even aerosolization to the lungs. There are currently over a dozen commercially available drugs encapsulated into nanoemulsions. Small oil droplet sizes and the associated stability of these products are critically important.
Oil-in-water emulsions with nano-sized droplets (nanoemulsions) are widely used in the pharmaceutical industry, for example, as an intravenous source of fatty acids when oral nutrition is disadvantageous or as bioactive compound carriers (e.g. drugs, vaccines). Pharmaceutical nanoemulsions can be administered by almost all available routes including parenteral, ocular, nasal, oral, topical, and even aerosolization to the lungs. There are currently over a dozen commercially available drugs encapsulated into nanoemulsions. Small oil droplet sizes and the associated stability of these products are critically important.
Current production method
Pharmaceutical nanoemulsions are currently produced by high-pressure homogenization (HPH). This method requires a coarse dispersion with droplets of about 1-10 µm in diameter to be prepared first (e.g. by a rotor-stator colloid mill), which is then pulled into a chamber and forced at an extremely high pressure (on the order of 1000 bars) through a narrow valve or split into two streams that go through separate micro-channels and subsequently collide with each other at very high velocities, producing droplet shear. This process is very energy-intensive and utilizes high-cost, large-footprint equipment that requires frequent and complex maintenance, is difficult to clean and service, and needs major redesign in order to enable aseptic processing.
The high-intensity ultrasonic method
High-amplitude ultrasonic emulsification is an attractive alternative, frequently used in laboratory studies. The challenge for this method, however, has been bridging the gap between laboratory research and its industrial implementation. Due to limitations of conventional ultrasonic technology, scaling up has not been possible without significant reduction in ultrasonic amplitudes, diminishing the intensity of cavitation-generated shear forces and compromising product quality. This limitation has been overcome with Barbell Horn Ultrasonic Technology (BHUT), which permits constructing industrial-scale processors operating at high ultrasonic amplitudes. Watch this video for more information.
Laboratory, bench and industrial-scale ultrasonic emulsification
A pharmaceutical nanoemulsion was manufactured by our R&D team using LSP-600 (laboratory, non-BHUT), BSP-1200 (bench, BHUT) and ISP-3600 (industrial, BHUT) high-amplitude ultrasonic processors. The prepared formulation was identical to that of the commercially produced MF59 squalene oil-based nanoemulsion currently used in vaccines against influenza and pandemic infections.
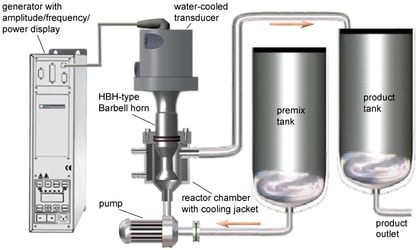 The LSP-600 processor was utilized in the batch mode: 40 ml samples were processed in a beaker with a temperature control jacket. The BSP-1200 and ISP-3600 processors were utilized in the single-pass flow-through mode (schematic on the left). The temperature in all experiments was maintained at 50 oC, and the ultrasonic amplitude was 90 microns. Prior to sonication, the formulation components were vigorously stirred together for 10 min using an overhead mixer. During the flow-through experiments, the components were continuously stirred in the premix tank. The target (desired) nanoemulsion mean droplet size (MDS, measured by dynamic light scattering) was 250 nm.
The LSP-600 processor was utilized in the batch mode: 40 ml samples were processed in a beaker with a temperature control jacket. The BSP-1200 and ISP-3600 processors were utilized in the single-pass flow-through mode (schematic on the left). The temperature in all experiments was maintained at 50 oC, and the ultrasonic amplitude was 90 microns. Prior to sonication, the formulation components were vigorously stirred together for 10 min using an overhead mixer. During the flow-through experiments, the components were continuously stirred in the premix tank. The target (desired) nanoemulsion mean droplet size (MDS, measured by dynamic light scattering) was 250 nm.
Experimental results
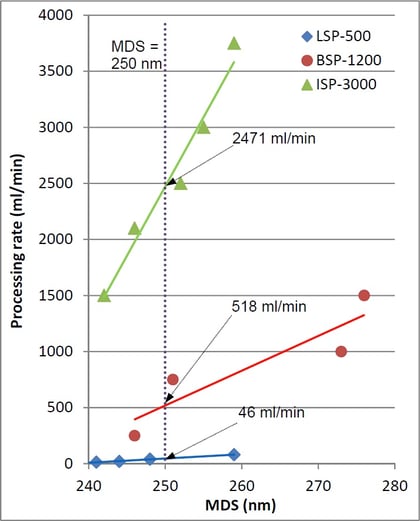 The target MDS was obtained at all three scales. Plots presented on the left were used to approximate the optimum processing rates corresponding to the target MDS at each scale (plot intersections with the vertical line at MDS = 250 nm), yielding the values of 46 ml/min, 518 ml/min and 2471 ml/min for the LSP-600, BSP-1200 and ISP-3600 processors, respectively.
The target MDS was obtained at all three scales. Plots presented on the left were used to approximate the optimum processing rates corresponding to the target MDS at each scale (plot intersections with the vertical line at MDS = 250 nm), yielding the values of 46 ml/min, 518 ml/min and 2471 ml/min for the LSP-600, BSP-1200 and ISP-3600 processors, respectively.
The productivity gain factors resulting from BHUT-enabled laboratory-to-bench and bench-to-industrial scale-up procedures, therefore, were ~11 and ~5, respectively, and the overall scale-up factor achieved by utilizing BHUT was ~55.
Currently, the MF59 nanoemulsion is manufactured using HPH (Microfluidizer M7250, industrial processor) at the rate of 1.5 L/min per pass with a total 5 passes required, resulting in the final productivity of about 300 ml/min. The productivity afforded by the ISP-3600 is significantly greater, while the equipment costs and processing complexity are much lower. Studies are currently underway to ensure that the quality of the ultrasonically produced nanoemulsions is acceptable for clinical applications.
.jpg?width=1994&height=332&name=Logo%20Sonomechanics%20White%20No%20Shadow%20R_Final%20(1).jpg)

