 One of the primary concerns when formulating oil-in-water (O/W) nanoemulsions is the stability of the finished product. Destabilization of an O/W nanoemulsion can occur due to several physical, chemical or biological processes that deteriorate its quality over time. Understanding and avoiding these processes is paramount to ensuring that products based on cannabis extract nanoemulsions (a.k.a. "water-soluble" CBD and THC) have a long and predictable shelf-life. In this post, common nanoemulsion destabilization pathways will be described, along with the most effective strategies to avoid them.
One of the primary concerns when formulating oil-in-water (O/W) nanoemulsions is the stability of the finished product. Destabilization of an O/W nanoemulsion can occur due to several physical, chemical or biological processes that deteriorate its quality over time. Understanding and avoiding these processes is paramount to ensuring that products based on cannabis extract nanoemulsions (a.k.a. "water-soluble" CBD and THC) have a long and predictable shelf-life. In this post, common nanoemulsion destabilization pathways will be described, along with the most effective strategies to avoid them.
Oil phase separation due to creaming/sedimentation, flocculation and/or Ostwald ripening
Oil phase separation from an O/W nanoemulsion can occur 1) when buoyancy or gravitational forces acting on suspended oil droplets make them float/sink (creaming/sedimentation) and, therefore, come into close proximity to each other, 2) due to attractive interactions between the droplets (flocculation) and/or 3) as the result of larger droplets' growth at the expense of smaller ones (Ostwald ripening).
1. Creaming/sedimentation and flocculation
For inadequately formulated or under-processed nanoemulsions with oil droplets larger than 200 - 300 nm, a mechanism known as creaming takes place when the buoyancy force causes the droplets (having a lower density than the continuous water phase) to rise to the top. In less common situations where the dispersed oil phase density exceeds that of water, the droplets sink due to gravitational forces - a process referred to as sedimentation. As droplets collect at the top or the bottom, they come into close proximity with each other and coalesce (combine with each other) until complete oil phase separation occurs. Another possible consequence of inadequate formulation is flocculation (droplet agglomeration), which occurs when initially dispersed oil droplets gather into clusters as the result of insufficient droplet repulsion. Flocculation is generally followed by creaming and coalescence after the clusters become large enough.
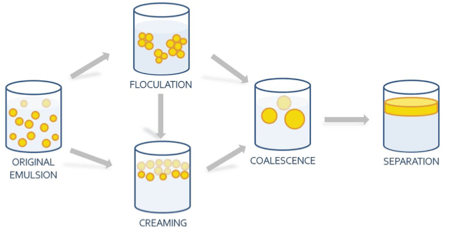
| Figure 1. Primary nanoemulsion destabilization pathways: creaming and flocculation. |
2. Ostwald ripening
Smaller oil droplets experience higher Laplace pressure (the difference in pressure between the inside and the outside of a curved surface), which increases their contents' solubility in the aqueous (water) phase. The result is a secondary, yet frequently encountered nanoemulsion destabilization mechanism known as Ostwald ripening that occurs when oil molecules from smaller oil droplets (with relatively higher Laplace pressure and oil solubility in water) dissolve into the aqueous phase and precipitate onto larger oil droplets (with relatively lower Laplace pressure and oil solubility in water). Under this mechanism, therefore, larger droplets grow at the expense of smaller ones until creaming begins to occur, ultimately leading to coalesce and phase separation.
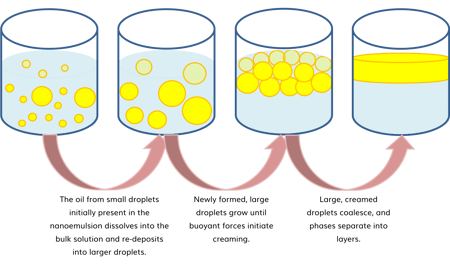
| Figure 2. Secondary nanoemulsion destabilization pathway: Oswald-ripening. |
3. Prevention techniques
When creaming/sedimentation, flocculation and Ostwald-ripening processes are properly mitigated, nanoemulsion destabilization and the resulting phase separation can generally be avoided. The table below lists some of the possible instability causes along with the associated destabilization mechanisms and recommended preventative measures:
| Cause of Instability | Mode of Destabilization | Recommendation |
| A. Insufficient shear intensity was applied during processing (e.g., stirring, shaking, using a hand-blender, ultrasonic bath, rotor-stator homogenizer or low-amplitude ultrasonic processor). | Median droplet size (d50) is likely to exceed 200 - 300 nm. Creaming or sedimentation destabilization pathways are significant due to high sensitivity of large droplets to buoyancy and gravitational forces. |
Utilize equipment able to provide greater shear intensity, such as a high-amplitude ultrasonic liquid processor, to reduce the median droplet size (d50) to well below 200 nm. |
|
B. Surfactant(s) was (were) not incorporated into the nanoemulsion or its (their) type(s), amount(s) and proportions (if more than one) were not correctly optimized. |
Flocculation pathway is significant due to insufficient inter-droplet repulsion. Likely followed by creaming or sedimentation due to high droplet cluster sensitivity to buoyancy and gravitational forces. |
Modify the formulation, using optimized surfactant type(s), cumulative amounts and ratios. Alternatively, take advantage of a commercially available all-in-one optimized formulation, such as NanoStabilizer®. |
|
C. Carrier oil was not incorporated or an incorrect carrier oil type was used. |
Ostwald-ripening pathway is significant due to excessive oil-phase solubility in water. Likely followed by creaming or sedimentation due to high sensitivity of large droplets to buoyancy and gravitational forces. |
Incorporate a carrier oil with low aqueous solubility (e.g., LCT). Alternatively, take advantage of a commercially available all-in-one optimized formulation, such as NanoStabilizer®. |
Cannabis extract-containing O/W nanoemulsions that have been processed and formulated properly are known to exhibit long-term kinetic stability - they can be considered indefinitely stable against all physical pathways leading to phase separation. This being said, chemical and biological degradation pathways may still be at play, as described below:
Oxidative Degradation of Cannabinoids
 It is well documented that many cannabinoids, including cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC), can undergo oxidative degradation upon exposure to air (oxygen) and light (UV) [1]. This remains true for cannabinoids incorporated into nanoemulsions. It is, therefore, recommended that finished cannabis extract-containing nanoemulsions be kept in dark, air-tight containers during storage. Additionally, antioxidants (e.g., tocopherols, carotenoids, terpenes) can be incorporated into the formulations to further limit oxidative degradation.
It is well documented that many cannabinoids, including cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC), can undergo oxidative degradation upon exposure to air (oxygen) and light (UV) [1]. This remains true for cannabinoids incorporated into nanoemulsions. It is, therefore, recommended that finished cannabis extract-containing nanoemulsions be kept in dark, air-tight containers during storage. Additionally, antioxidants (e.g., tocopherols, carotenoids, terpenes) can be incorporated into the formulations to further limit oxidative degradation.
Microorganism Activity
As in many water-based products, microbial growth in nanoemulsions is a potential shelf life-limiting pathway. If a microorganism (e.g., fungus) is allowed to exist and proliferate in a water-based product, it may reduce its potency and even make it harmful. Processing under aseptic conditions can help circumvent this issue, but this is generally an excessively expensive approach. Luckily, it is easy and inexpensive to eliminate the presence of microorganisms in a nanoemulsion by sterile filtration using a hydrophilic filter membrane with 220 nm pores. It is, however, important to point out that all droplets in the nanoemulsion must be below 220 nm for this to be effective, otherwise filter clogging and material loss is likely to occur. This typically requires the nanoemulsion to be at least somewhat translucent.

The filtration step performed after the nano-emulsification process carried out with the BSP-1200 ultrasonic processor is demonstrated on the left. For further information, see: "Why Sterile-Filter Water-Compatible Cannabis Extract Nanoemulsions?"
Although sterile filtration ensures that the finished product does not contain microorganisms, once a nanoemulsion container is opened (for instance, by the end-user), the product becomes exposed to the microorganisms in the atmosphere. To mitigate the effect of this exposure, cannabis extract nanoemulsions, as most water-based products, should contain small amounts of preservatives.
Storing Nanoemulsions
In order to protect nanoemulsions from light, oxygen and microbial contamination, they should be sterile-filtered and stored in dark-glass, air-purged, autoclaved and sealed containers. Unless frozen or boiled, nanoemulsions tend to be stable in a wide temperature range, however, refrigeration is recommended as an additional precaution against microorganism activity.
Conclusion
All nanoemulsion destabilization pathways can be avoided by correctly formulating, processing and storing these products. To enable companies in the pharmaceutical, nutraceutical and food & beverage industries to manufacture their own high-quality nanoemulsions, Industrial Sonomechanics (ISM) offers high-intensity ultrasonic liquid processors with unsurpassed nano-emulsification capabilities at laboratory, bench and industrial scales. Our equipment clients who do not have the capability or desire to develop and optimize their own formulations and processing procedures can also take advantage of all-in-one NanoStabilizer®. When used in conjunction with ISM ultrasonic processors, this blend of food-grade (GRAS) carrier oils, emulsifiers, and preservatives (all derived from natural sources) makes it possible to easily and quickly produce highly translucent, permanently stable and fully water-compatible nanoemulsions with median droplet sizes (d50) in the range of 10 – 50 nanometers. Lastly, ISM also provides laboratory and industrial-scale inline sterilizing filters that make finished product sterilization simple and fast.
Have questions? Please do not hesitate to contact us or leave a comment below.
References
[1] Irenne Gabriela Trofin, Gabriel Dabija, Danut-Ionel Vaireanu, Laurentiu Filipescu. Long - term Storage and Cannabis Oil Stability, Revista De Chimie - Bucharest - Original Edition, 63 (3), (March 2012).
.jpg?width=1994&height=332&name=Logo%20Sonomechanics%20White%20No%20Shadow%20R_Final%20(1).jpg)


